26. Gratz, A. J., P. Bird, and G. B. Quiro (1990) Dissolution of quartz in aqueous basic solution, 106-236° C: Surface kinetics of "perfect" crystallographic faces, Geochimica et Cosmochimica Acta, 54, 2911- 2922.
Abstract. Past work on dissolution kinetics of minerals has focused on particulates and the total transfer of material to solution. We look instead at local topography of single crystals. Etching of quartz in alkali hydroxide solutions produces four types of features: flat-bottomed negative crystals with facets for walls, believed to nucleate on microfractures; jumbo pits, giant etch pits cored by dislocations and often with hollow cores; small pits, also believed to be dislocation-cored; and etch tunnels extending from some jumbo pits. A transition from selective etching (revelation of intrinsic defects) to nonselective etching (most intrinsic defects dissolving at the same rate as bulk crystal) occurs as a function of ionic strength, with small pits forming only at ionic strengths below ~0.005 m. This transition is attributed to the decrease in Debye-Hückel length with increasing solution strength.
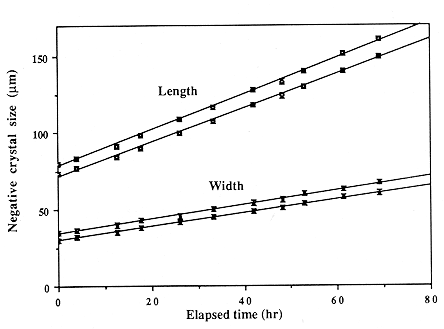
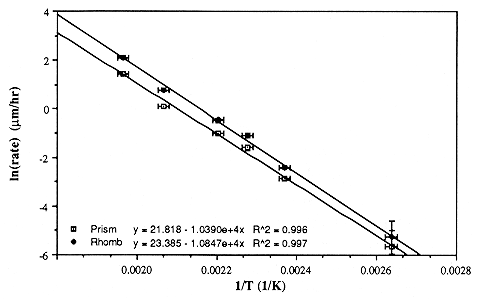
We introduce the negative crystal method, whereby dissolution rates of nominally perfect crystal faces are obtained by measuring the size of individual negative crystals during a sequence of dissolution steps. Rates are obtained to ~4% accuracy, with temperature control of ± 1.25° C. Using this method, we find apparent activation energies for dissolution of the prism and for an average of the rhombs to be 86.39 and 90.19 kJ/mol, respectively, with corresponding pre-exponential factors of 2.99 ´ 109 and 1.43 ´ 1010 m m/h. The closeness of these two activation energies argues for a single atomic site controlling the rate, while site density varies with the crystal orientation. At a constant ionic strength of 0.01 m, the rates vary in a complex manner with hydroxide activity, increasing as activity to the ~0.5 power up to ~0.005 m, above which the rate is nearly independent of the alkalinity. Very similar etching behavior is observed in all KOH-KCl mixtures, as well as in LiOH, NaOH, RbOH, and CsOH, although the detailed morphology of small pits is controlled by the chemistry of the etchant. The lower atomic weight hydroxides have nearly identical etch rates; RbOH and CsOH dissolve quartz roughly twice as quickly. We also note a factor of ~1.6 enhancement in dissolution rate in certain synthetic over natural samples.